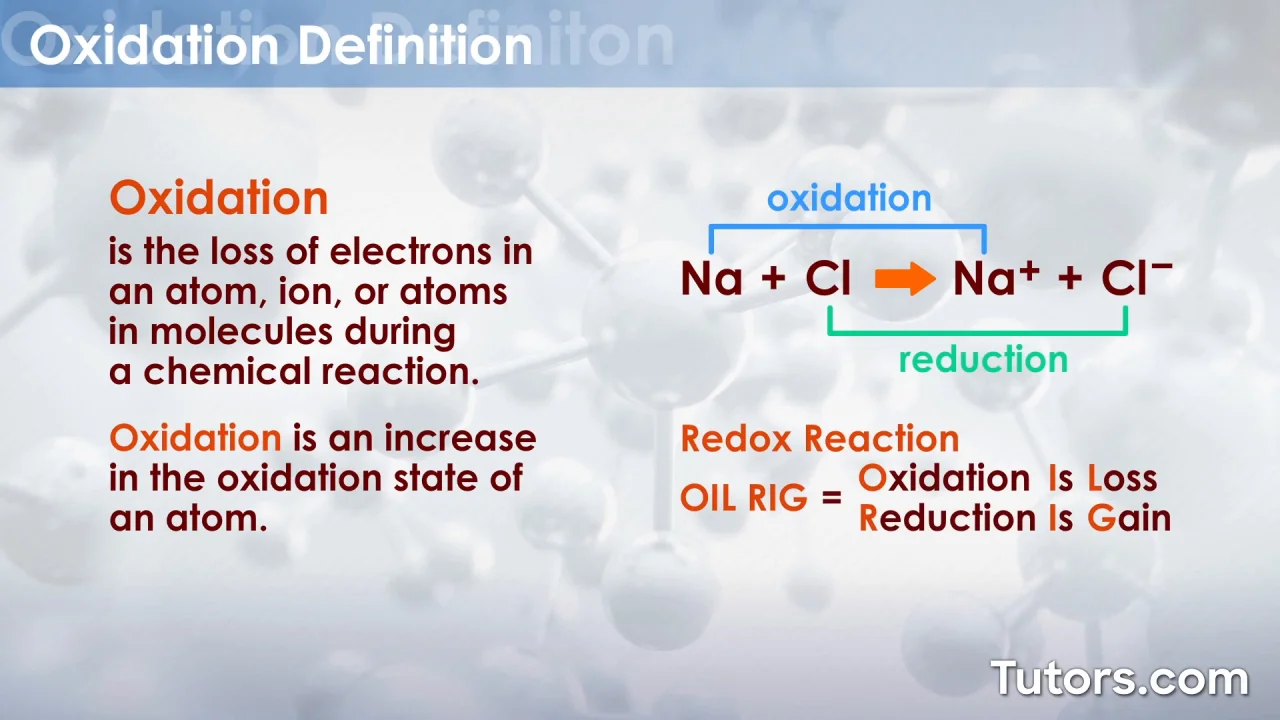
Oxidation Is Loss Reduction Is Gain = OILRIG. This is a helpful acronym to describe the term given to a loss or gain of electrons. If atom looses or gains an electron it becomes an ion, and the term to describe this process depends on whether an atom has lost (oxidation) or gained (reduction) an electron. For example, if Chlorine (Cl) becomes.. 4 Li (s) + O 2 (g) 2 Li 2 O (s) The state symbols in brackets show the physical state of. the substance at the reaction temperature. Solid (s), liquid (l), gas (g), or dissolved in water (aq). aq is called aqueous which comes. from the Latin word aqua meaning water. If you do not know the state of a substance.
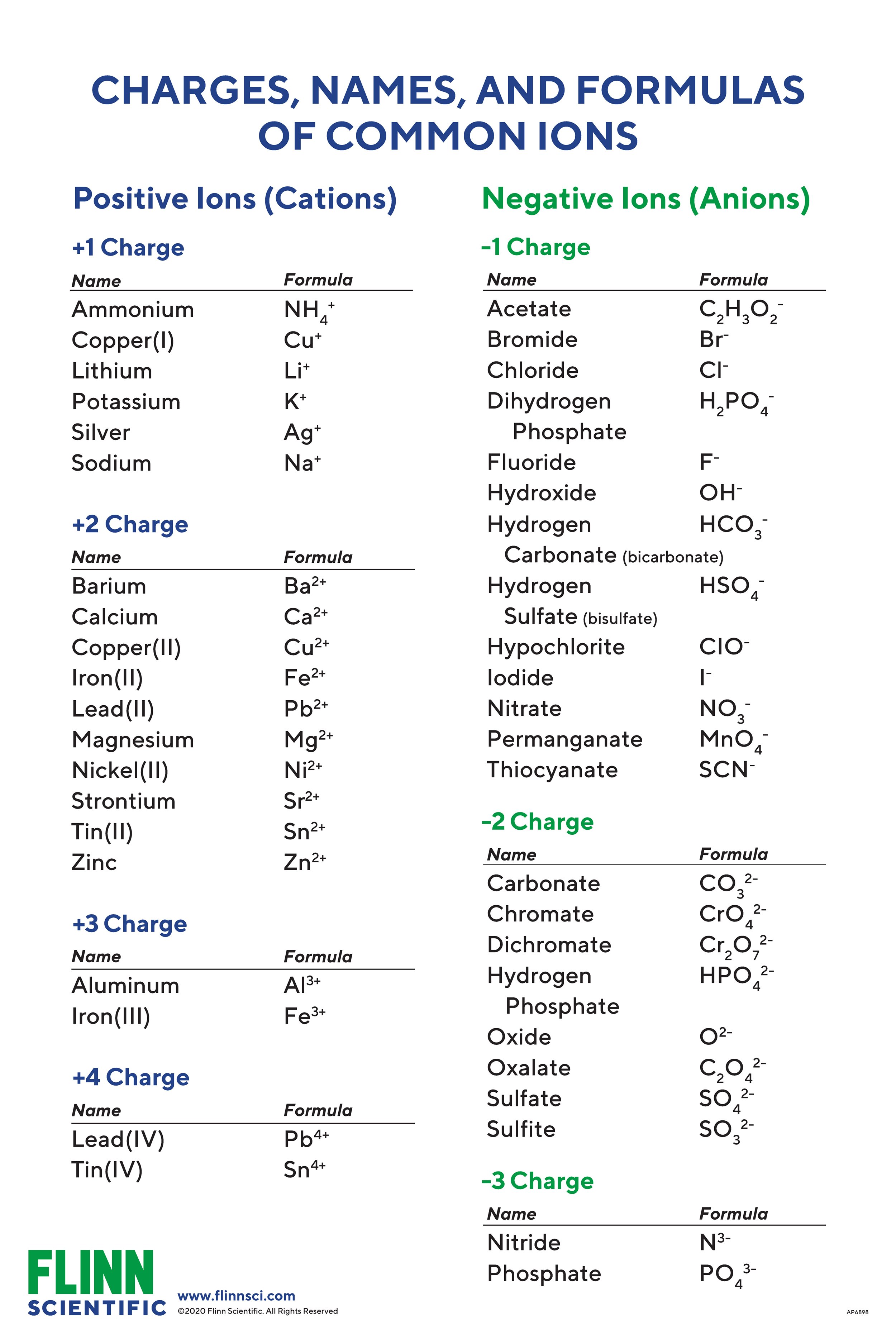
Ion Names, Formulas and Charges Chart Flinn Scientific
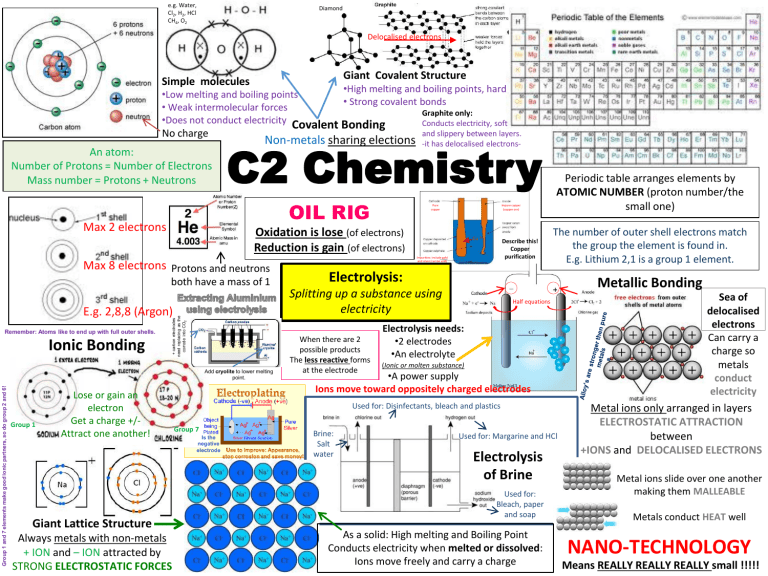
C2 Chemistry Revision poster
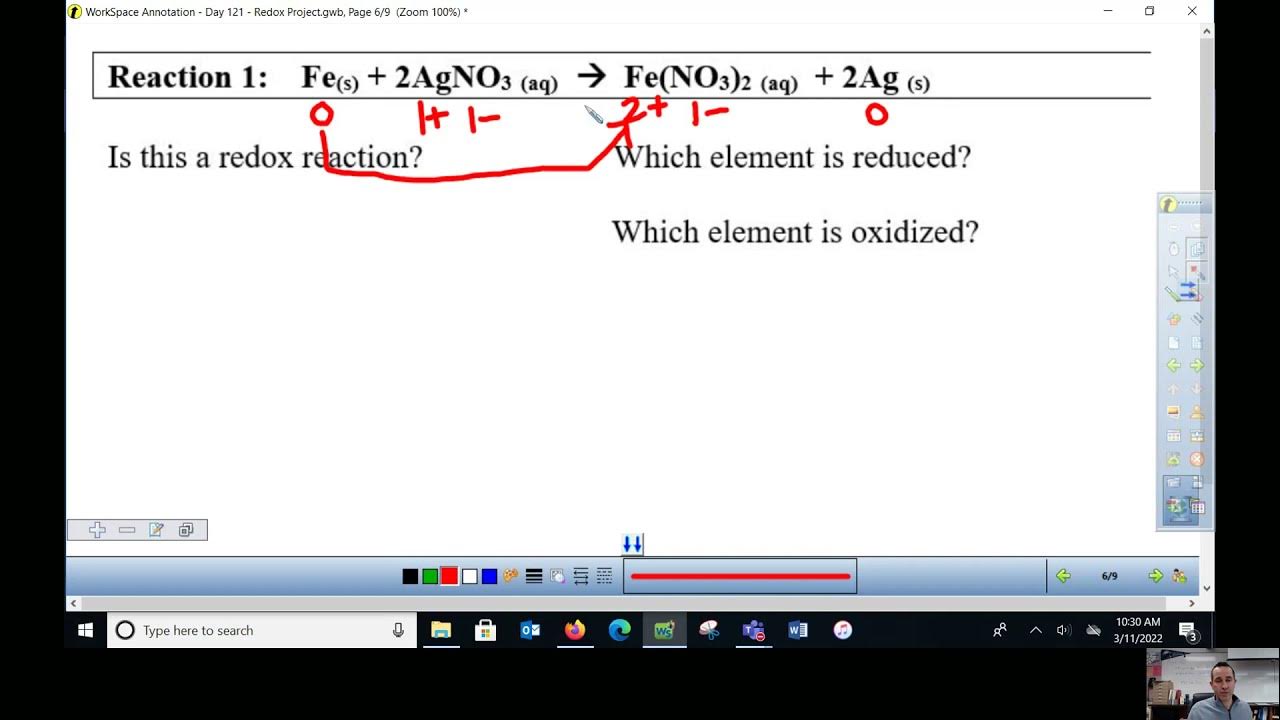
Oxidation Reduction Overview OIL RIG Chemistry YouTube
APChemistryatGWHS
PPT Redox Reactions PowerPoint Presentation, free download ID2204000
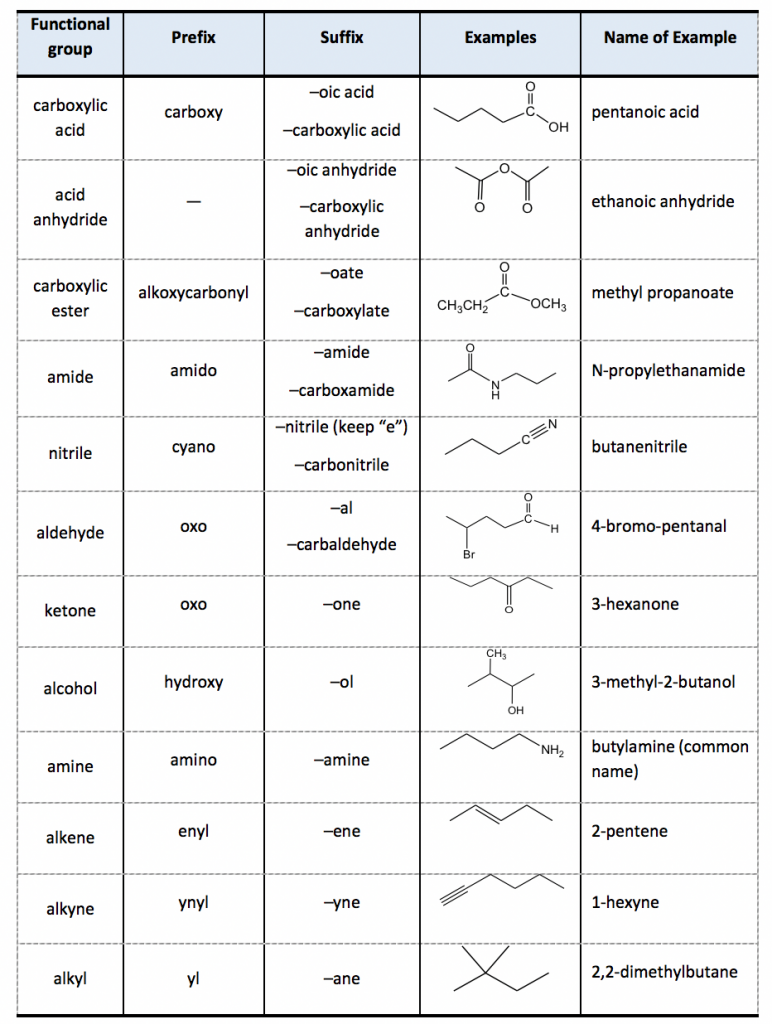
![Functional Groups in Organic Chemistry [Infographic] Functional Groups in Organic Chemistry [Infographic]](https://chemistry.com.pk/wp-content/uploads/2014/08/Functional-Groups-in-Organic-Chemistry.png)
Functional Groups in Organic Chemistry [Infographic]

6.1.1 Reduction & Oxidation Edexcel A Level Chemistry Revision Notes 2017 Save My Exams
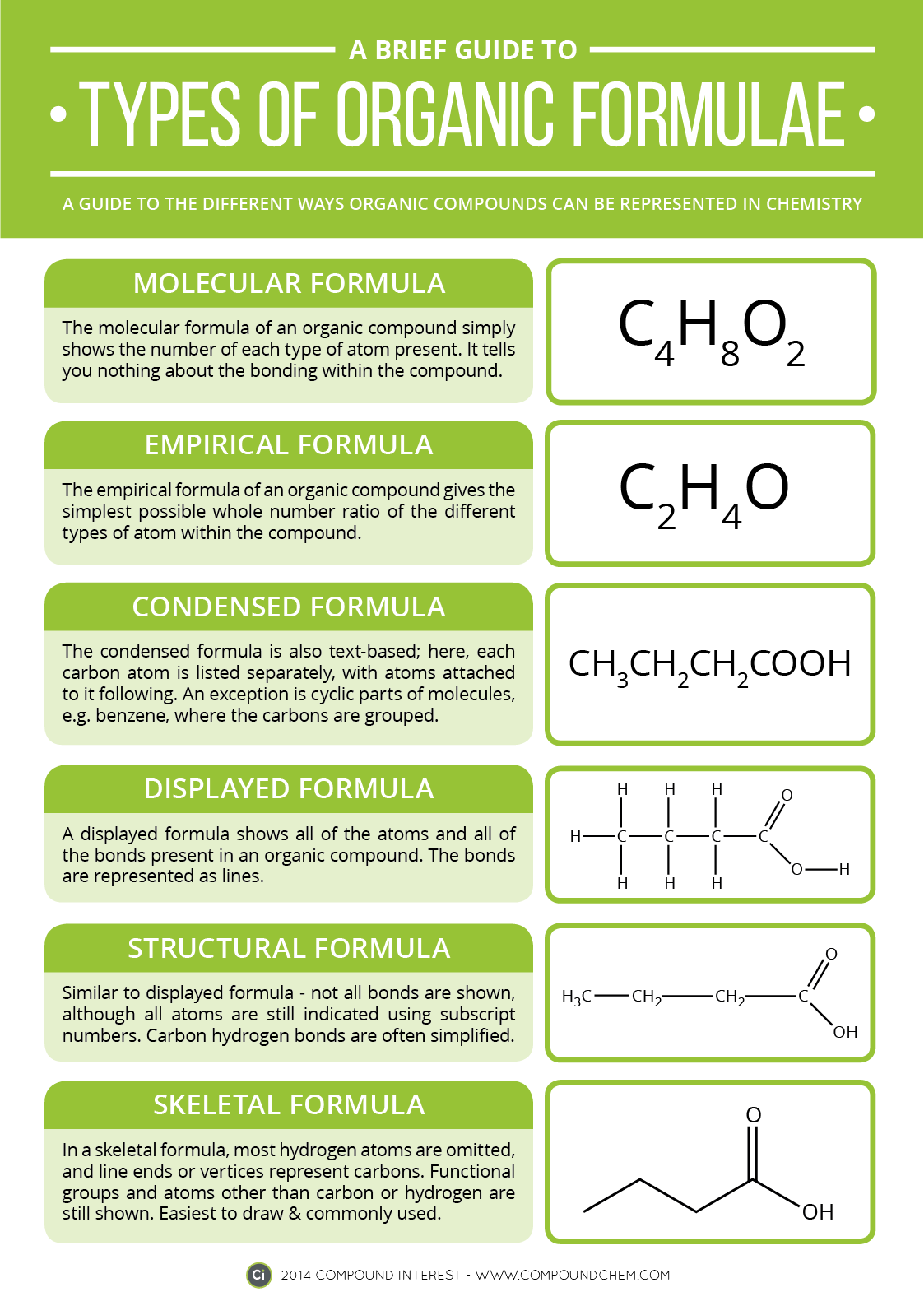
A Brief Guide to Types of Organic Chemistry Formulae

Classifying Chemical Reactions Stacy Goldstein Library Formative

Schematic of Oil rig Download Scientific Diagram

Periodic Table Of Elements Timeline
/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)
Solved (e) On The Reaction Energy Diagram Belo 114

Appendix Periodic Table of the Elements The Basics of General, Organic, and Biological Chemistry
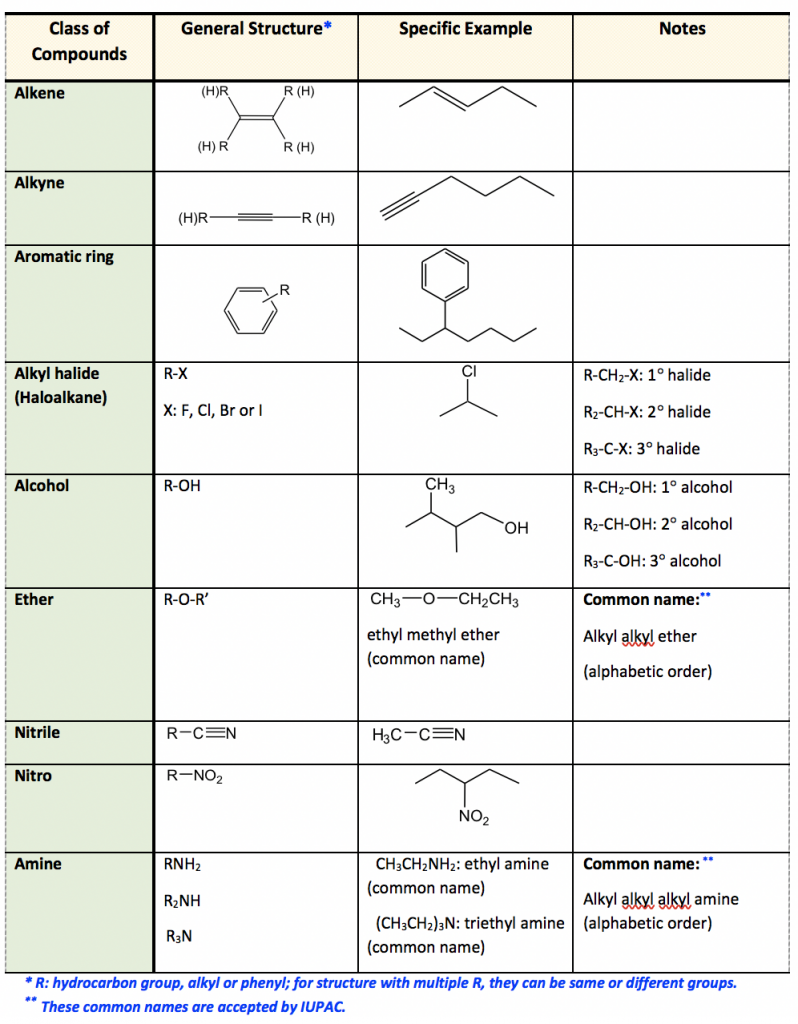
Which of the Following Compounds Contains an Ether Functional Group
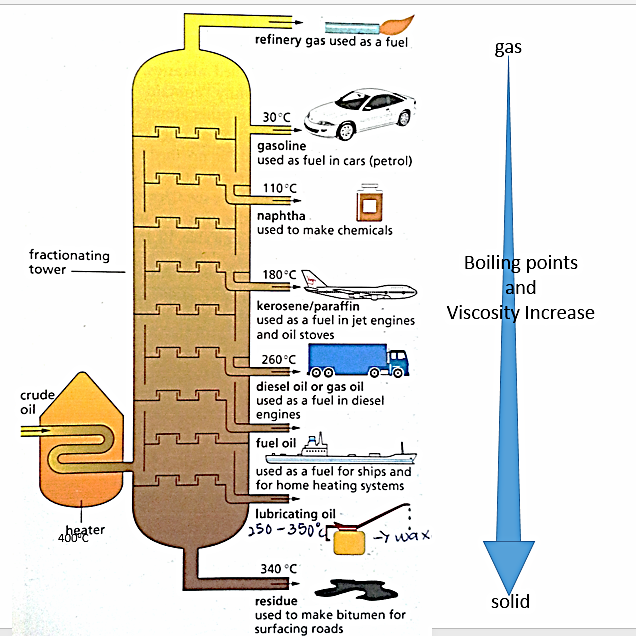
O Level Chemistry Oil Refining Crude Oil
Oxidation Chemistry

Free Download Organic Chemistry (9th Global Ed.) By Leroy G. Wade Jr. & Jan William Simek
/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
Ken de soorten chemische reacties (met voorbeelden)
Ir Spectrum Table Of Compounds Elcho Table

PPT Chemistry Chapter 8 Chemical Equations PowerPoint Presentation, free download ID3973749
oxidation is the loss of electrons reduction is the gain of electrons Balance the half equation for the formation of aluminium during electrolysis: Al 3+ + e-→ Al. The balanced half equation is.. Oxidation and Reduction with respect to Electron Transfer. Oxidation is loss of electrons. Reduction is gain of electrons. In addition to "LEO roars GER" (loss of electrons - oxidation, gain of electron - reduction), another convenient acronym is : Na Na+ +e− (4.2.1) (4.2.1) Na Na + + e −. - sodium is oxidized to the +1 oxidation state.