ATAGI advises that the Pfizer bivalent BA.4/5 vaccine can be used as a booster dose by adolescents and adults aged ≥12 years who are recommended to receive a COVID-19 booster according to the ATAGI 2023 booster advice. The Pfizer bivalent BA.4/5 vaccine is not currently registered for use in children aged <12 years or as a primary series .. Immunogenicity and safety of a bivalent (omicron BA.5 plus ancestral) SARS-CoV-2 recombinant spike protein vaccine as a heterologous booster dose: interim analysis of a phase 3, non-inferiority, randomised, clinical trial. A bivalent omicron-containing booster vaccine against Covid-19. N Engl J Med. 2022; 387: 1279-1291. View in Article.
Asesores de la FDA autorizar vacuna contra covid19 de Pfizer para niños entre 5 y

Cone Health Now Offering COVID19 Bivalent Boosters WXLV

Second Omicron Booster Authorized MedPage Today

US authorises the use of dualvariant covid19 vaccine boosters New Scientist

FDA authorizes bivalent COVID19 boosters for children ages 5 to 11

Pfizer's looking like good protection against severe illness from BA.4&5 CoronavirusMa

FDA Authorizes OmicronTargeted Booster Shots SOMEONE SOMEWHERE

SPIKEVAX Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran) COVID19 Vaccine with

FDA authorizes omicron COVID booster shots for BA.4, BA.5
Antibody response from Omicron BA.4/BA.5 bivalent booster no better than original vaccine
Updated COVID19 bivalent booster shots now available in Coachella Valley
RACGP Second bivalent booster receives ATAGI
COVID19 Vaccine Appointments Don's Pharmacy

Pfizer Reports New Bivalent Booster Is More Effective Against Current Omicron Variants Gazette
BioNTech SE on Twitter "A booster dose of our Omicron BA.4/BA.5adapted bivalent COVID19
Moderna’s bivalent COVID vaccine for 18 and above MOH

Covid le vaccin adapté à Omicron BA.4BA.5 désormais disponible

Cone Health to offer COVID19 bivalent booster vaccines
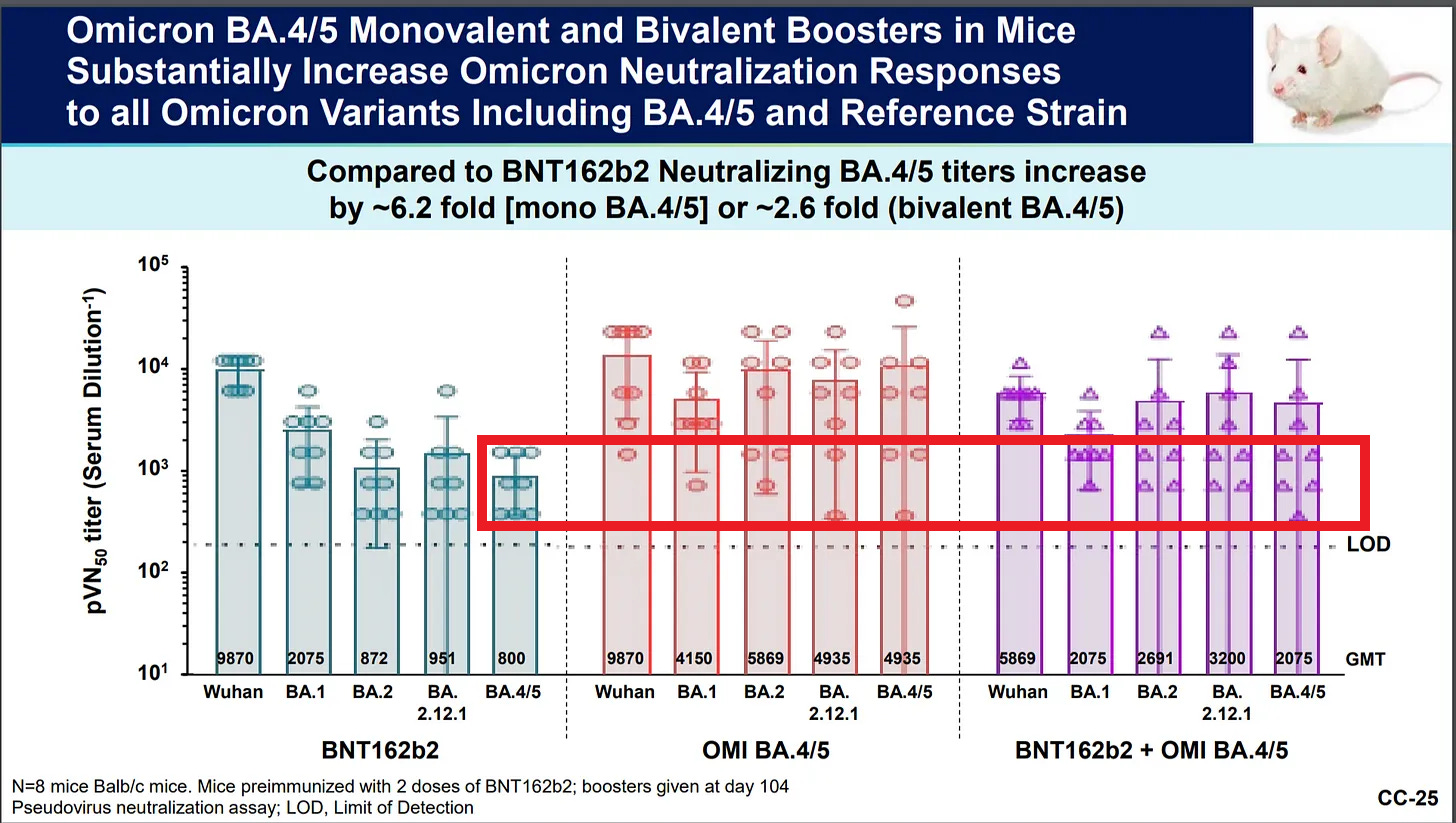
Why Did Pfizer Choose the Bivalent “Booster” over the “Monovalent” Booster?

FDA to consider Pfizer omicron booster for kids under 5
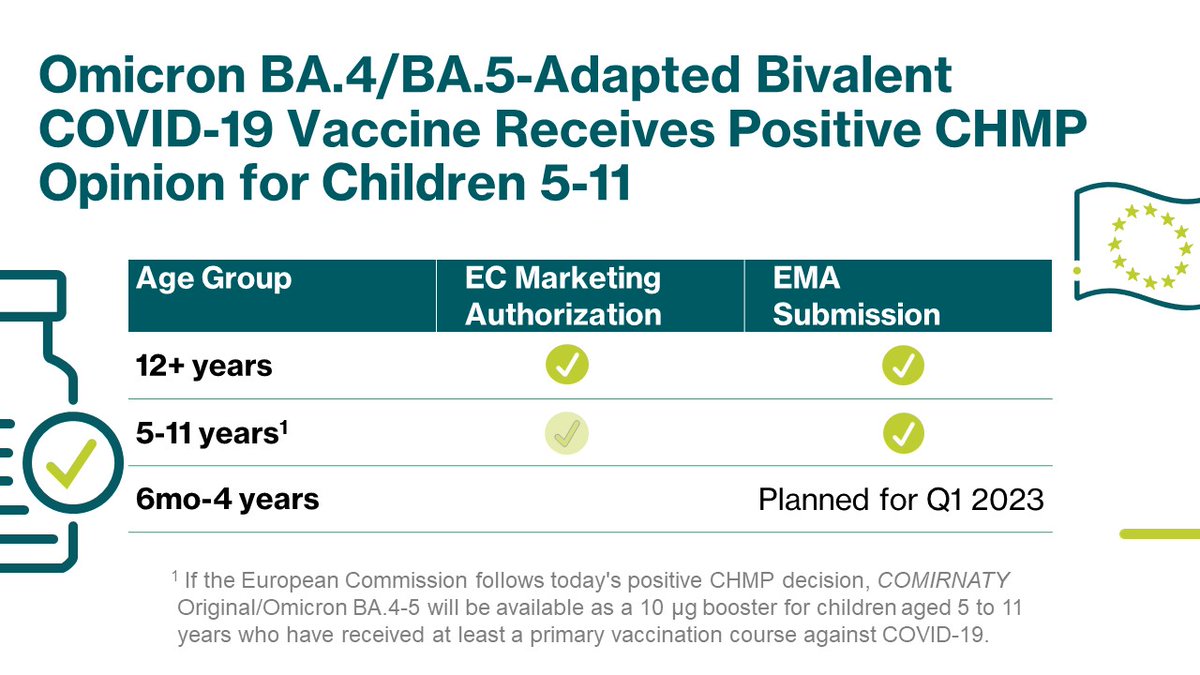
The research cohorts included 3,368,697 adults vaccinated with the fourth dose, of which 1,290,999 were bivalent BA.4-5-vaccinated, 992,282 were bivalent BA.1-vaccinated, and 1,085,416 were.. Pfizer-BioNTech Comirnaty ® Original and Omicron BA.4/BA.5, bivalent COVID-19 vaccine. The Pfizer-BioNTech Comirnaty ® Original and Omicron BA.4/BA.5, bivalent COVID-19 vaccine is approved as a primary series for people who are 6 months of age and older and as a booster for people who are 5 years of age and older. Its safety and effectiveness.